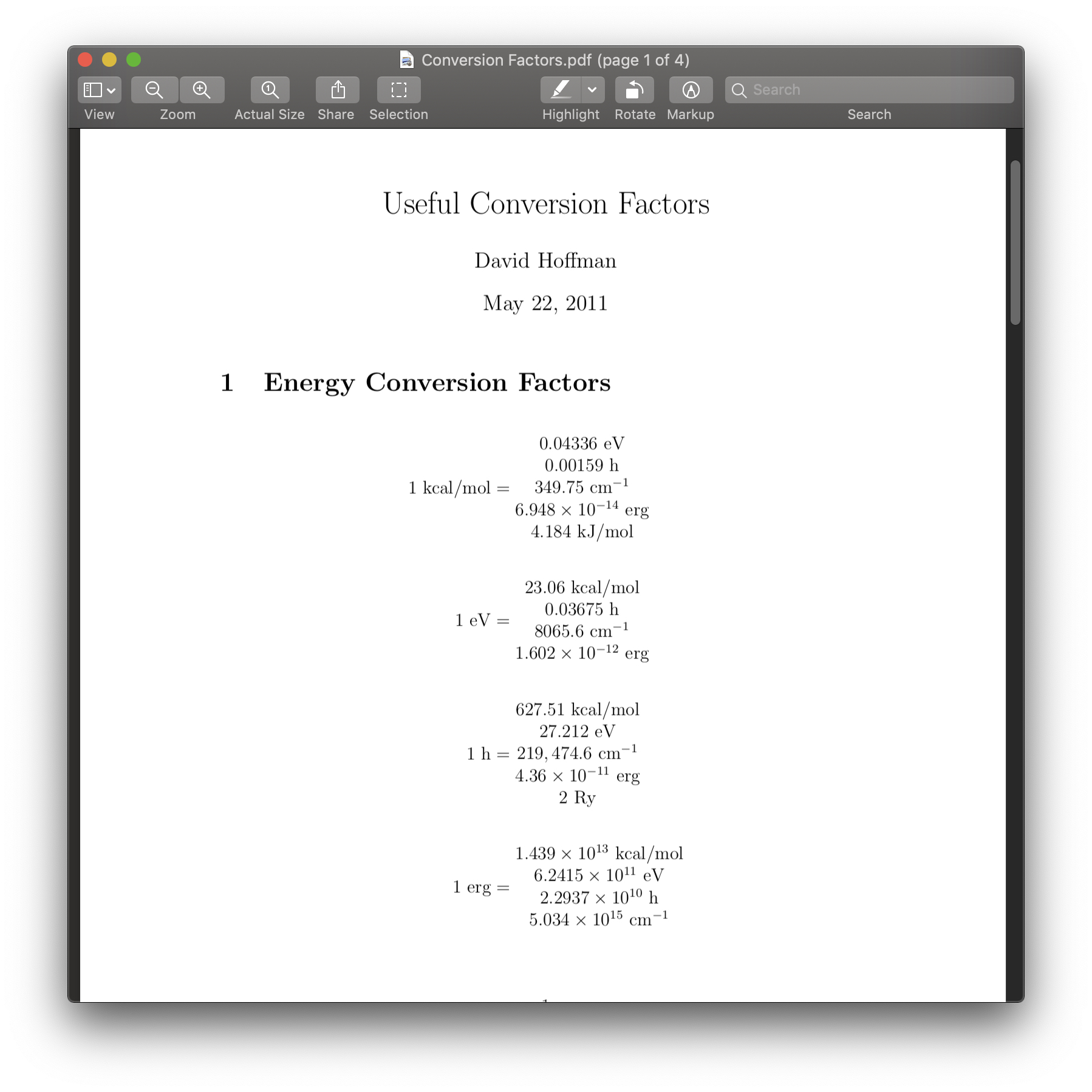
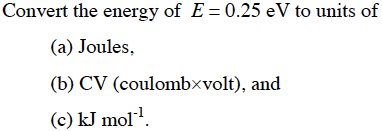SOLVED: (a)how much energy in KJ/mol is released when an electron makes a transition from n=5 to n=2 in a hydrogen atom?
the energy required to convert all atoms presrent in 1.2g magnesium to magmnesium to mg^2+ ions if lE,and lE_2 of magnesium are 120kj mol^ 1 and 240 kj mol^ 1 respectivel

SOLVED: Calculate (a) the energy (in eV) of a 5.3-Ã… X-ray photon and (b) the energy (kJ/mol) of a 530-nm photon of visible radiation. Given the following constants and unit conversion factors:

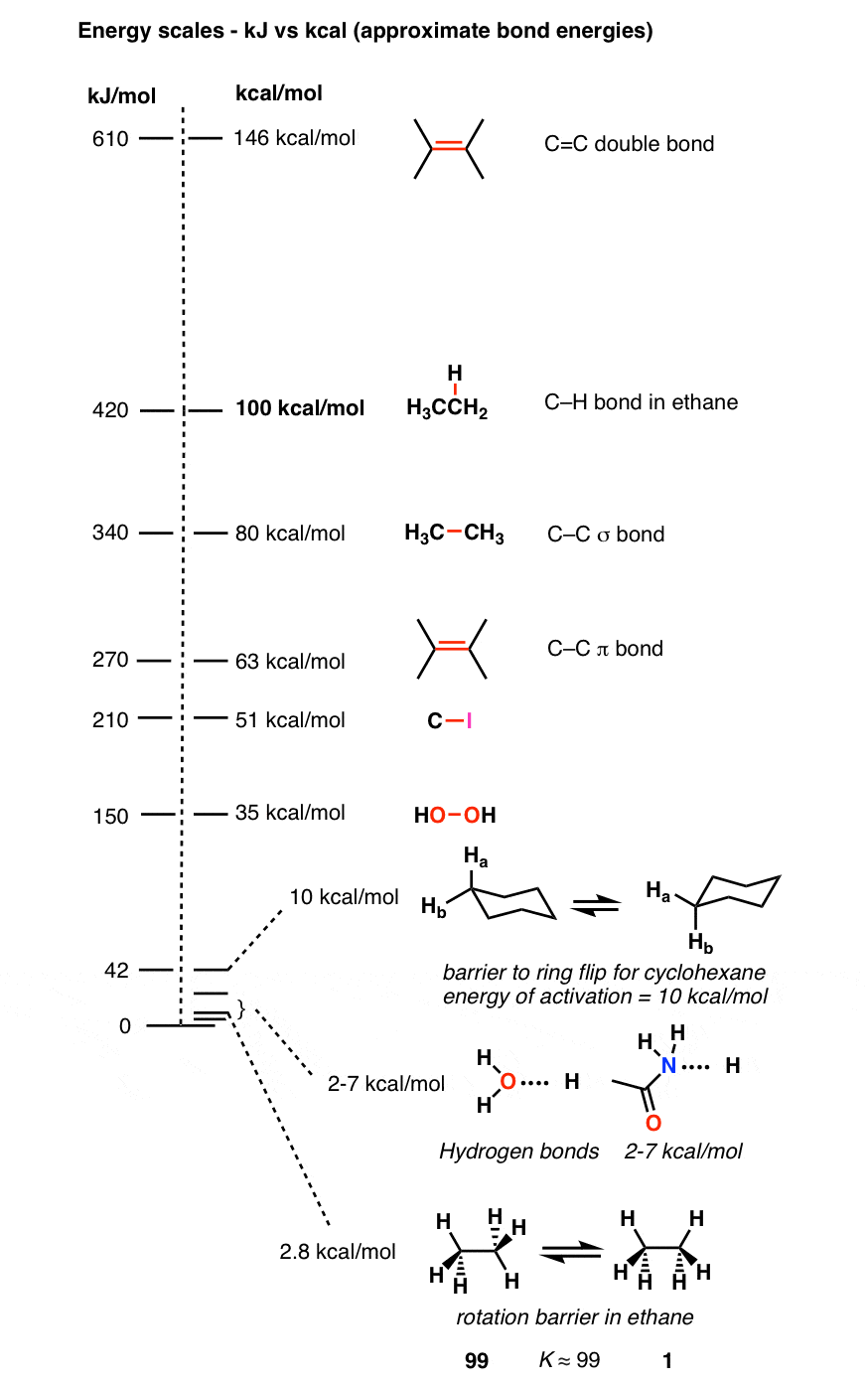
1 MODELING MATTER AT NANOSCALES 6. The theory of molecular orbitals for the description of nanosystems (part II) The Hartree-Fock method applied. - ppt download
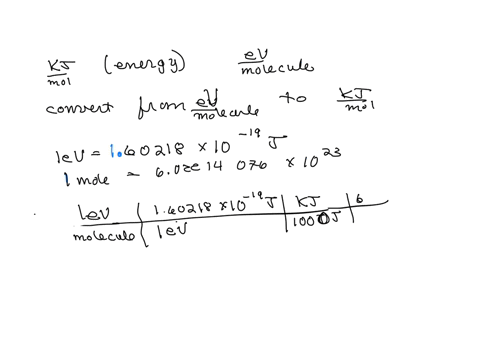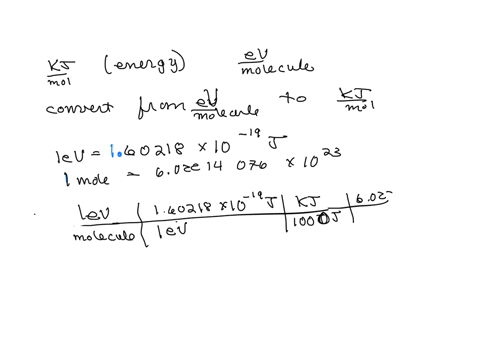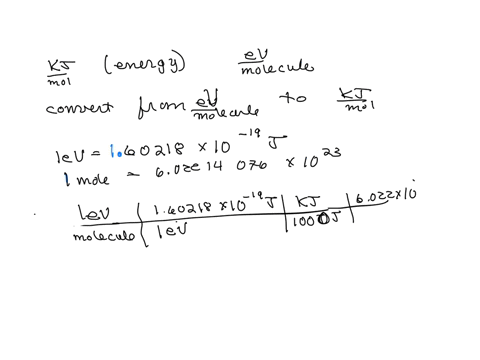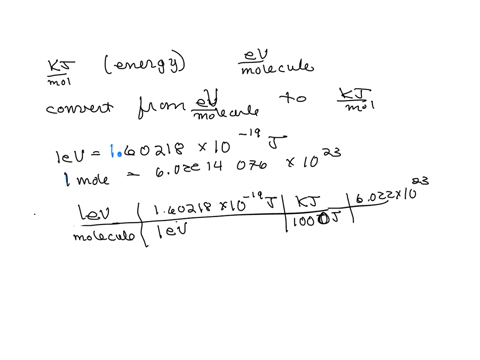
SOLVED: The energy associated with chemical processes is often quoted in kilojoules per mole of reactants. Please derive the conversion factor from eV/molecule to kJ/mol. (1 eV = 60218 × 10-19 J, 1 mole = 6.02214076 × 1023).

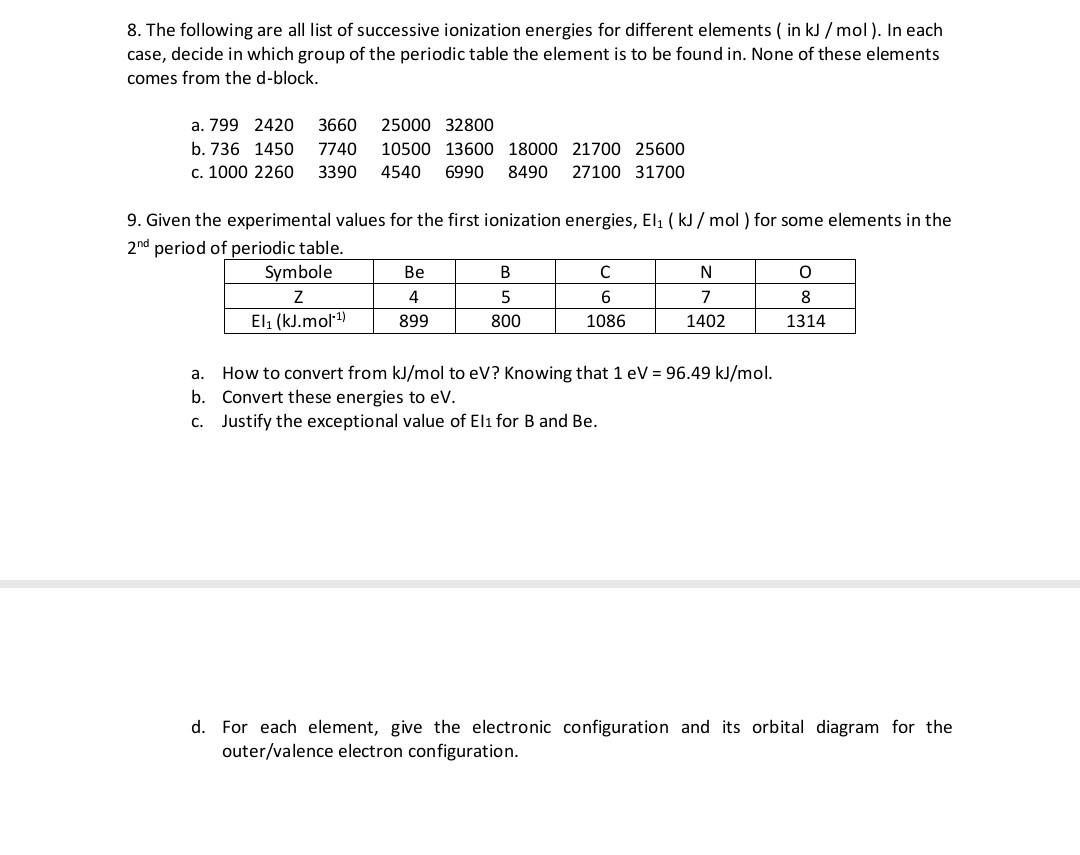
First and second ionisation energies of magnesium are 7.646 and 15.035eV respectively. The amount of energy in kJ needed to convert all the atoms of magnesium into Mg^{2+} ions present in 12
Magnesium has the firšt and second ionizatio: potential 7.646 and 15.035 ev respectively. What is theof energy required to convert all the magnesium atoms to Mg2+ ions present in 24 mg to

Week 1: Basics Reading: Jensen 1.6,1.8,1.9. Two things we focus on DFT = quick way to do QM – 200 atoms, gases and solids, hard matter, treats electrons. - ppt download